SpineGuard (FR0011464452 – ALSGD), an innovative company that designs, develops and markets disposable medical devices designed to make spine surgery safer, announced today the world’s first spinal fusion surgery performed with the one-step-insertion of pedicle “smart screws” guided by DSG™ (Dynamic Surgical Guidance) technology. This surgery was performed successfully by Dr. Court and his team at the Centre Hospitalier Universitaire Bicêtre Paris-Sud in France. Dr. Tropiano in Marseille, France, and Dr. Bolger in Ireland have scheduled surgeries in the coming days. For SpineGuard and Neuro France Implants, who announced their co-development partnership in early 2015, this successful first surgery performed immediately after obtaining CE mark is a critical milestone in preparation for the commercial launch of the G2S pedicle screw instrumentation that integrates the DSG technology.
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20160105006573/en/
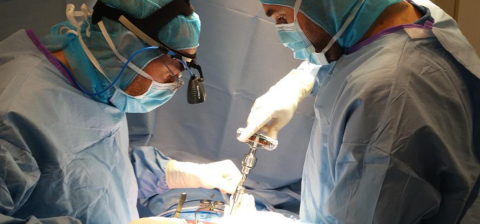
“The direct insertion of pedicle screws along with real-time x-ray-free feedback represents a key step for spine surgery,” said Charles Court, M.D., French orthopedic surgeon. (Photo: Business Wire)
“The direct insertion of pedicle screws along with real-time x-ray-free feedback represents a key step for spine surgery. This first case gave me a glimpse of the great potential of this innovation in terms of safety and precision. A multi-centric study will allow us to substantiate it,” said Charles Court, M.D., orthopedic surgeon at the Centre Hospitalier universitaire Bicêtre Paris-Sud in France.
Pierre Jérôme, CEO and co-founder of SpineGuard, said: “This pioneering surgery validates the clinical utility of embedding our DSG technology into the vertebral implant itself, thus enabling its insertion without any preliminary step. I believe this major technological advancement will further secure and simplify the most commonly performed instrumented spine procedure, fusion.”
Patrice Moreau, CEO and co-founder of Neuro France, added: “For 17 years Neuro France Implants has been focusing on simplifying and securing spine surgery through innovative implants. This first direct insertion of our G2S pedicle screws integrating the DSG technology is a real success, our collaboration with SpineGuard is bearing fruit.”
Pedicle screw-based fusion has become the gold standard for treating spine instabilities and deformities. The market continues to grow globally due to the increasing number of patients requiring surgical treatment, a larger number of surgeons being trained, and technological advancements such as minimally invasive surgery, bone substitutes and dynamic stabilization. Worldwide there are about one million pedicle screw-based procedures performed annually1. However, accuracy of pedicle screw placement remains a critical issue in spine surgery. In recently published papers studying screw placement accuracy, the average rate of misplaced screws is approximately 20%, with 2-11% of patients presenting neurologic or vascular complications due to misplaced screws2.
More information on the DSG™ technology, its new applications and surgeons’ testimonials here.
Previous release: 45% of growth for 3Q 2015 revenue.
Next
Press Release: 2015 FY Sales on January 14, 2016.
SpineGuard
will participate in the 'Invest Securities Biomed' conference on
January 27, 2016, in Paris.
About SpineGuard®
Co-founded in 2009 in France and the USA
by Pierre Jérôme and Stéphane Bette, SpineGuard’s mission is to make
spine surgery safer. Its primary objective is to establish its
proprietary DSG™ (Dynamic Surgical Guidance) technology as the global
standard of surgical care, initially for safer screw placement in spine
surgery and then in other surgeries. PediGuard®, the first device
designed using DSG was co-invented by Maurice Bourlion, Ph.D., Ciaran
Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. It is
the world’s first and only handheld device capable of alerting surgeons
to potential pedicular or vertebral breaches. Over 40,000 surgical
procedures have been performed worldwide with PediGuard. Numerous
studies published in peer-reviewed medical and scientific journals have
demonstrated the multiple benefits that PediGuard delivers to patients,
surgical staff and hospitals. In 2015, SpineGuard started to expand the
applications of DSG into pedicle screws through partnerships with
innovative surgical companies in France and the US. SpineGuard has
offices in San Francisco and Paris. For further information, visit www.spineguard.com.
Disclaimer
The SpineGuard securities may not be offered or
sold in the United States as they have not been and will not be
registered under the Securities Act or any USA state securities laws,
and SpineGuard does not intend to make a public offer of its securities
in the United States. This is an announcement and not a prospectus, and
the information contained herein does and shall not constitute an offer
to sell or the solicitation of an offer to buy, nor shall there be any
sale of the securities referred to herein in the United States in which
such offer, solicitation or sale would be unlawful prior to registration
or exemption from registration.
1 Source : I-Data Research
2 Source : Bibliography
View source version on businesswire.com: http://www.businesswire.com/news/home/20160105006573/en/






